Introduction
O2 Lewis structure is one of those chemistry ideas that sounds complicated at first, but once you slow it down, it becomes surprisingly clear. In this guide, we’ll walk through the O2 Lewis structure step by step, using simple explanations, clear diagrams, and everyday comparisons to make everything stick.

What Is the O2 Lewis Structure?
The O2 Lewis structure is a simple drawing that shows how two oxygen atoms share electrons to form a molecule. Think of it as a map that explains who shares what and why. Each oxygen atom brings six valence electrons to the table. Together, they share electrons in a covalent bond so both atoms feel “complete.”
A Lewis structure uses dots to represent electrons and lines to show shared pairs. For O2, the drawing reveals a diatomic molecule with a double bond between the two oxygen atoms. This visual explanation helps us understand why oxygen behaves the way it does in air, fire, and life itself.

If you ever struggled with the structure meaning of chemical drawings, this concept clears that up. Just like emojis show emotions quickly, a Lewis structure shows bonding fast and clearly. Once you get it, reading chemical diagrams becomes much easier.
O2 Lewis Structure Setup
Setting up the O2 Lewis structure is like preparing ingredients before cooking. You need to know how many electrons you have and where they belong. Oxygen sits in group 16 of the periodic table, which tells us it has six valence electrons. Since O2 has two oxygen atoms, that gives us twelve valence electrons total.
The goal is to arrange these electrons so each oxygen atom follows the octet rule, meaning eight electrons in its outer shell. To do this, oxygen atoms share electrons rather than steal them. That sharing creates a strong covalent bond.

This setup explains why oxygen naturally exists as a pair instead of single atoms floating around. The structure is stable, balanced, and easy to represent in a clean diagram. Once the setup is clear, the drawing becomes almost automatic.
Count Valence Electrons
Each oxygen atom has six valence electrons. Two oxygen atoms mean twelve valence electrons total. This number guides every step of the O2 Lewis structure and keeps the diagram accurate.

Form the Initial Bond
Start by placing the two oxygen atoms side by side. Connect them with a single line to show an initial shared electron pair. This bond is only the beginning.

Create the Double Bond
To satisfy the octet rule, oxygen atoms share two pairs of electrons. This creates a double bond, shown as two lines between the atoms in the Lewis structure.
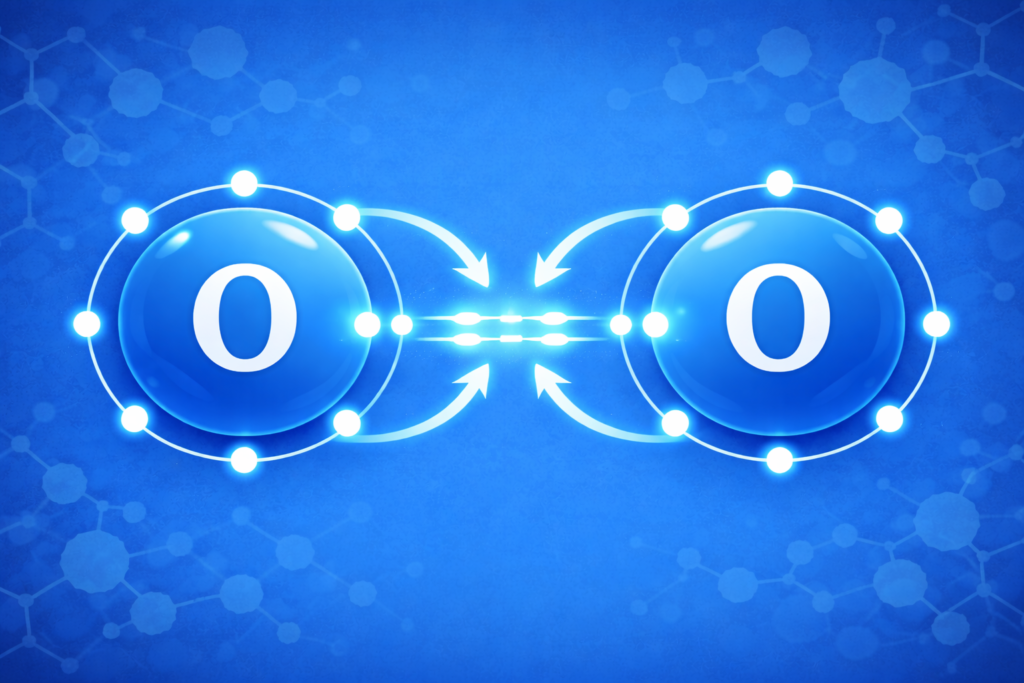
Final O2 Setup
Each oxygen ends up with two lone pairs and a double bond between them. This final setup gives both atoms eight electrons, creating a stable molecule.

Why the O2 Lewis Structure Is Important
The O2 Lewis structure is important because it helps turn an invisible molecule into something we can clearly understand. Instead of guessing how oxygen behaves, the Lewis structure gives a visual explanation of how electrons are shared and arranged. This simple diagram explains why oxygen exists as a diatomic molecule, why it is stable in the air, and why it reacts so easily in fires, rusting, and living cells. In short, the structure connects tiny electrons to big real-world effects, making chemistry feel logical rather than abstract.

Predicts Shape
The O2 Lewis structure shows two oxygen atoms connected by a double bond in a straight line. Because only two atoms are involved, the molecule is linear. This clear shape helps us predict how oxygen fits into reactions and how it moves and interacts with other molecules in gases and compounds.
Explains Reactions
By showing a double covalent bond, the Lewis structure explains why oxygen is reactive but not unstable. The shared electrons can be rearranged during reactions, which is why oxygen easily forms water, carbon dioxide, and metal oxides during combustion and corrosion.
Reveals Properties
The electron arrangement shown in the Lewis structure helps explain oxygen’s key properties, such as its ability to support burning and its paramagnetic behavior. These properties come directly from how electrons are shared and distributed between the two atoms.
Basic Concepts You Need Before Drawing O2
Before you draw the O2 Lewis structure, it’s important to understand a few basic ideas. These concepts act like simple rules of the road. Once you know them, the structure of oxygen feels logical and easy, not confusing. If you ever feel lost while learning chemistry, reviewing these basic concepts first makes everything smoother and more intuitive.

What is an Atom?
An atom is the smallest unit of an element that still keeps its identity. It has a nucleus in the center and electrons moving around it. Every substance around us is made of atoms. Understanding atom basics is the first step to understanding how molecules like O2 form.
Valence Electrons
Valence electrons are the electrons found in the outer shell of an atom. These electrons matter the most because they take part in bonding. Oxygen has six valence electrons, which explains why it needs to share electrons when forming the O2 molecule.
Octet Rule
The octet rule says that atoms want eight electrons in their outer shell to feel stable, similar to having a full set. Oxygen follows this rule closely. In O2, both oxygen atoms share electrons so each one reaches this stable eight-electron arrangement.
Chemical Bonds
A chemical bond forms when atoms connect by sharing or transferring electrons. In O2, the bond is covalent, meaning electrons are shared equally. This sharing creates a strong link between the two oxygen atoms and keeps the molecule stable in nature.
Lewis Dot Structure Steps
- Count the total valence electrons
- Write the element symbols
- Place electrons as dots around atoms
- Form bonds by sharing electrons
- Check that each atom follows the octet rule
Once these ideas are clear, drawing the O2 Lewis structure becomes a simple, step-by-step process instead of a guessing game.
How to Draw the O2 Lewis Structure Step by Step?
Drawing the O2 Lewis structure is a lot like building something with clear instructions. If you follow the steps in order, you won’t get stuck, and you won’t need to guess. The main goal is simple: place the electrons so both oxygen atoms feel “complete” (meaning they each reach an octet). Oxygen is a diatomic molecule, so you’re only working with two atoms, which makes it a perfect practice example.
One helpful way to think about this is like two people splitting a shared playlist. Each person wants a “full set” of songs, so they agree to share a couple of tracks. In chemistry, those shared “tracks” are electron pairs. When oxygen atoms share two pairs, they form a double covalent bond. The final diagram is clean, balanced, and easy to recognize.

As you go through these steps, keep your total electron count in mind the whole time. That’s the most common place people slip up. Also, remember that lines in a Lewis structure represent shared electron pairs, while dots represent lone pairs that stay on one atom.
Count Valence Electrons
Each oxygen atom has 6 valence electrons. Since there are two oxygen atoms in O2, you add them together: 6 + 6 = 12 total valence electrons. Write “12” down first, because this number controls the entire drawing and keeps your diagram accurate.
Sketch the Atoms
Write the symbol O twice with a little space between them: O O. This sketch is your basic frame. Oxygen atoms are the same, so there’s no “central atom” choice here. You’re simply showing two identical atoms ready to connect.
Fill Outer Shells
Start placing electrons as dots around the atoms. Add lone pairs first, then single dots if needed. Keep counting until you’ve placed all 12 valence electrons. At this stage, you may notice each oxygen still doesn’t have a full octet—and that’s your clue that bonding must change.
Form Double Bond
Connect the two oxygen atoms with a line to represent a shared electron pair. If a single bond doesn’t give both atoms an octet, convert one lone pair from each oxygen into another shared pair. Now you’ll have two lines between the atoms: O=O, showing the double bond.
Final Check
Check two things: (1) You used exactly 12 valence electrons total, and (2) each oxygen has 8 electrons around it (counting shared electrons in the bond). In the final O2 Lewis structure, each oxygen has two lone pairs, and the double bond sits between them.
Properties of O₂
The properties of oxygen (O₂) are closely connected to its Lewis structure and electron arrangement. Because oxygen forms a stable diatomic molecule with a double covalent bond, it shows a unique mix of stability and reactivity. These properties explain why oxygen is essential for life, supports fire, and plays a major role in both natural and industrial processes. Looking at O₂ through its physical and chemical properties helps connect the simple Lewis diagram to how oxygen behaves in the real world.

Physical Properties
| Property | Description |
| State | Gas at room temperature |
| Color | Colorless |
| Odor | Odorless |
| Molecular type | Diatomic |
| Magnetism | Paramagnetic |
Oxygen’s physical properties make it easy to mix with air and essential for breathing. Its paramagnetic nature comes from unpaired electrons, which adds an extra layer of interest beyond the Lewis structure.
Chemical Properties
| Property | Description |
| Bond type | Covalent double bond |
| Reactivity | Highly reactive |
| Combustion role | Supports burning |
| Oxidation ability | Strong oxidizing agent |
These chemical properties explain why oxygen reacts easily with many elements. The shared electrons in its double bond allow oxygen to take part in reactions that release energy and form vital compounds.
Does O₂ Follow the Octet Rule?
Yes, O₂ does follow the octet rule, and this is one of the reasons it is such a useful example when learning Lewis structures. The O₂ Lewis structure clearly shows how both oxygen atoms arrange their electrons to reach a stable configuration. By sharing electrons instead of gaining or losing them, each oxygen atom ends up with a full outer shell. This balanced setup explains why oxygen naturally exists as a diatomic molecule and remains stable in the air we breathe. Understanding these structure rules helps learners see that chemistry follows clear patterns rather than random behavior.
What is the Octet Rule?
The octet rule states that atoms tend to be most stable when they have eight electrons in their outer shell. This rule is based on the electron arrangement of noble gases. Many elements, including oxygen, follow this rule by sharing electrons in covalent bonds.

O₂ Molecule Structure
In the O₂ molecule, two oxygen atoms bond together by sharing two pairs of electrons. This creates a double bond between them. The Lewis structure shows this clearly with two lines between the atoms, helping both reach a stable outer shell.
Electron Count in O₂
Each oxygen atom starts with six valence electrons. By sharing two electron pairs in the double bond, each atom effectively counts eight electrons around it. This confirms that O₂ fully satisfies the octet rule without breaking any stability principles.
Understanding the Double Bond in O₂
The double bond in the O₂ Lewis structure is the heart of why oxygen behaves the way it does. It explains oxygen’s stability, its reactivity, and even why it plays such a powerful role in life and combustion. A single bond would not be enough for oxygen atoms to feel complete, while a triple bond would make the molecule too rigid. The double bond strikes the perfect balance. By sharing two pairs of electrons, both oxygen atoms reach a stable state while still remaining reactive enough to take part in chemical reactions. This balance is what makes oxygen both reliable and useful in nature.
What is a Double Bond?
A double bond forms when two atoms share two pairs of electrons instead of just one. In diagrams, it is shown as two lines between atoms. This type of bond is stronger than a single bond and helps atoms achieve a stable electron arrangement.

Why Oxygen Needs It?
Each oxygen atom has six valence electrons and needs two more to reach an octet. By forming a double bond, the two oxygen atoms share exactly the electrons they need. This allows both atoms to become stable without gaining or losing electrons.
Lewis Dot Picture
In the Lewis dot picture, the double bond appears as two lines between the oxygen atoms. Each oxygen also has two lone pairs shown as dots. This simple drawing clearly explains how electrons are shared and arranged in O₂.
Bond Strength
The double bond in O₂ is strong enough to keep the molecule stable under normal conditions. At the same time, it can break during reactions, releasing energy. This strength makes oxygen reliable yet reactive, which is essential for processes like breathing and burning.
Uses of Oxygen
Oxygen is one of the most useful elements on Earth, and its importance comes directly from how it is bonded and structured. The O₂ Lewis structure explains why oxygen is stable enough to exist freely in the air, yet reactive enough to power life, industry, and technology. Because oxygen forms a strong covalent bond and exists as a diatomic molecule, it can safely travel through the atmosphere and then react when needed. From the air we breathe to rockets leaving Earth, oxygen quietly supports countless processes that shape everyday life.

Breathing and Life
Oxygen is essential for breathing and survival. Inside the body, oxygen helps cells release energy from food through respiration. This energy powers movement, thinking, and growth. Without a steady supply of O₂, cells cannot function, which is why oxygen is vital for all complex life.
Medical Help
In medicine, oxygen is used to support patients who struggle to breathe. Oxygen tanks, masks, and ventilators deliver extra O₂ to the lungs. This support helps people recover from illness, injury, or surgery and can be life-saving in emergencies.
Making Steel
Oxygen plays a major role in steel production. It is used to remove unwanted carbon and impurities from molten iron. This process improves the strength and quality of steel, making it suitable for buildings, vehicles, and tools used every day.
Welding and Cutting
Oxygen fuels extremely hot flames when combined with other gases. These flames are used for welding metals together and cutting through thick steel. The high heat comes from oxygen’s ability to support rapid combustion.
Space and Rockets
In space exploration, oxygen is used as an oxidizer in rocket engines. Liquid oxygen helps fuel burn efficiently in space, where air is absent. This makes oxygen essential for launching rockets and sustaining astronauts beyond Earth.
Lone Pairs in the O₂ Lewis Structure
Lone pairs are an important part of the O₂ Lewis structure, even though they are easy to overlook. While bonding electrons get most of the attention, lone pairs play a quiet but powerful role in shaping how oxygen behaves. In the O₂ molecule, not all valence electrons are used for bonding. Some remain as lone pairs, staying close to their original atom. These electrons affect how oxygen interacts with other substances, how close molecules can approach each other, and how reactions take place. Understanding lone pairs helps turn the Lewis structure from a simple drawing into a more complete explanation of oxygen’s behavior.
Role of Lone Pairs
Lone pairs are electrons that are not shared between atoms. In O₂, these electrons stay on each oxygen atom. They influence electron repulsion and help determine how oxygen reacts with other elements, even though they are not part of the bond itself.
How Many Lone Pairs?
Each oxygen atom in the O₂ Lewis structure has two lone pairs. That means there are four lone pairs total in the molecule. These lone pairs, along with the double bond, allow each oxygen atom to reach a full octet.

Why They Matter?
Lone pairs matter because they affect reactivity and chemical behavior. They help explain why oxygen can form strong bonds with other elements and why it behaves differently from molecules that have no lone pairs. Without them, the O₂ structure would be incomplete and misleading.
Common Mistakes When Drawing the O₂ Lewis Structure
Even though the O₂ Lewis structure is one of the simpler Lewis diagrams, mistakes are very common, especially for beginners. Most errors come from rushing or skipping basic steps like counting electrons or checking the octet rule. Because oxygen is a diatomic molecule with a double bond, it behaves a little differently from single-bonded molecules. Being aware of common mistakes helps you avoid confusion and build confidence. When drawn correctly, the O₂ Lewis structure is clean, balanced, and easy to recognize, but one small error can throw the entire diagram off.
Wrong Electron Count
A frequent mistake is counting the wrong number of valence electrons. Each oxygen atom has six valence electrons, giving a total of twelve for O₂. Forgetting this or miscounting leads to missing or extra electrons in the diagram.
Single Bond Only
Many people stop after drawing a single bond between the two oxygen atoms. A single bond does not give both atoms a full octet. Oxygen needs a double bond to satisfy the octet rule, so stopping too early creates an incorrect structure.
Ignoring Lone Pairs
Another common error is leaving out lone pairs. Each oxygen atom must have two lone pairs. Without them, the electron count is wrong, and the structure no longer represents how oxygen actually behaves.
Octet Rule Violation
Failing to check the octet rule is a final major mistake. In a correct O₂ Lewis structure, both oxygen atoms must have eight electrons around them. Always do a final check to confirm this before finishing your diagram.
Lewis Structure vs Molecular Orbital Theory for O₂
The O₂ Lewis structure gives a clear and simple picture of how oxygen atoms bond, but it does not tell the full story. To fully understand oxygen, especially its magnetic behavior, scientists also use Molecular Orbital (MO) Theory. These two models look at bonding from different angles. The Lewis structure focuses on shared electron pairs, while MO theory looks at how electrons spread across the entire molecule. O₂ is a classic example that shows why both models matter and how they complement each other in chemistry.
Lewis Structure Basics
Lewis structures use dots and lines to show valence electrons and bonds. In O₂, the Lewis structure shows a double covalent bond and lone pairs on each oxygen atom. This model is easy to draw and great for explaining bonding, stability, and the octet rule.
MO Theory Basics
Molecular Orbital theory treats electrons as shared across the whole molecule instead of belonging to one bond. In O₂, MO theory shows that some electrons remain unpaired. This explains why oxygen is paramagnetic, something the Lewis structure alone cannot show.
Key Differences
Lewis structures focus on localized electron pairs and simple diagrams. MO theory focuses on electron energy levels and orbitals spread across atoms. Lewis models are simpler, while MO theory gives deeper insight into properties like magnetism and bonding strength.
Why O₂ Shows the Gap?
O₂ highlights the limits of Lewis structures because it behaves in ways the model cannot predict, such as paramagnetism. MO theory fills this gap by explaining electron behavior more accurately, making oxygen a perfect example of why advanced models exist.
Real-World Importance of Oxygen’s Bonding Structure
Oxygen’s bonding structure is not just a classroom concept—it directly shapes the world we live in. The way two oxygen atoms share electrons in a stable double covalent bond explains why oxygen can exist freely in the atmosphere and still react when needed. The O₂ Lewis structure helps connect microscopic electron behavior to massive real-world effects, from breathing and weather to fire and industrial production. Without this specific bonding setup, oxygen would either be too reactive to remain in the air or too inactive to support life. Its balanced structure is what makes oxygen one of the most important elements on Earth.
Stable Air We Breathe
Oxygen’s double bond makes O₂ stable enough to exist in the atmosphere without constantly reacting. This stability allows oxygen to mix evenly with nitrogen, creating air that is safe to breathe and consistently available for living organisms across the planet.

Powers Life’s Energy
Inside living cells, oxygen plays a key role in releasing energy from food. Its bonding structure allows it to accept electrons during respiration, which helps produce the energy that powers movement, thinking, and growth in plants and animals.
Fuels Fire and Industry
Oxygen’s ability to react easily comes from its electron arrangement. This makes it essential for combustion. Fires, engines, and many industrial processes rely on oxygen’s bonding structure to release large amounts of energy efficiently and predictably.
Builds Water and Weather
Oxygen bonds with hydrogen to form water, one of the most important substances on Earth. This bonding drives rainfall, clouds, and weather systems. Without oxygen’s specific bonding behavior, Earth’s climate and water cycle would not exist as we know them.
How O₂ Lewis Structure Helps in Learning Chemistry Basics
The O₂ Lewis structure is often one of the first molecular diagrams students learn, and for good reason. It takes abstract ideas like electrons and bonds and turns them into something visual and logical. Instead of memorizing rules, learners can actually see how atoms share electrons and why molecules form the way they do. Because oxygen is simple yet powerful, its Lewis structure acts like a training ground for understanding larger and more complex molecules. Mastering this structure builds confidence and creates a strong foundation for future chemistry topics.
What It Shows?
The O₂ Lewis structure shows how electrons are arranged and shared between atoms. It clearly displays the double covalent bond and the lone pairs on each oxygen atom, helping learners understand where electrons go and how stability is achieved.
Octet Rule Basics?
This structure is a perfect example of the octet rule in action. Each oxygen atom reaches eight electrons by sharing pairs. Seeing the rule applied visually makes it easier to understand than reading about it in text alone.
Predicting Properties?
By looking at the Lewis structure, learners can predict properties like reactivity and bond strength. The double bond suggests stability, while the lone pairs hint at how oxygen might interact with other elements in reactions.
Steps to Draw It
The O₂ Lewis structure teaches a repeatable method: count electrons, place atoms, form bonds, and check the octet rule. These steps apply to many molecules, not just oxygen, making it a valuable learning tool.
Why It Helps Learning?
Because the O₂ Lewis structure is simple and familiar, it reduces confusion. It builds visual thinking skills and helps learners connect rules with real examples, making chemistry feel logical, approachable, and easier to remember.
Visual Tips to Remember the O₂ Lewis Structure
Remembering the O₂ Lewis structure becomes much easier when you turn it into pictures and stories. Visual tricks help your brain store information faster than plain facts. Instead of memorizing dots and lines, you connect the idea to familiar images. These small mental shortcuts are especially useful during tests or quick reviews, when you need to recall the structure without overthinking it. The goal is to make the diagram feel natural and obvious the moment you see “O₂.”
Picture Two Friends Holding Hands
Imagine two friends standing side by side, holding both hands. Each shared hand represents one shared pair of electrons. Because they’re holding two hands, you instantly remember the double bond. The friends stay balanced and connected, just like the two oxygen atoms in O₂.

Count Dots Like Candy
Think of valence electrons as candies you must share fairly. You start with twelve candies total. You give each oxygen atom enough candy so both end up with eight. This playful image helps you remember the total electron count and avoid missing or extra dots.
Octet Rule as Full Table
Picture a table with eight seats. An oxygen atom feels comfortable only when all eight seats are filled. Lone pairs and shared bonds are just different ways of filling those seats. If a seat is empty, you know the structure isn’t finished yet.
Sketch Shortcut Shape
When in doubt, remember the shortcut: O=O. Two O’s with two lines between them instantly bring back the full Lewis structure. Using familiar symbols and easy examples like this keeps the diagram locked in your memory without stress.
Frequently Asked Questions
The O₂ Lewis structure often raises quick but important questions, especially for learners who want clarity without overcomplicating things. This FAQ section answers the most common doubts in simple language, using short explanations that reinforce what you’ve already learned. Think of it as a final review that helps lock the concept into memory and clears up confusion before moving on to more complex chemistry topics.
The correct Lewis structure for O₂ shows two oxygen atoms connected by a double bond. Each oxygen atom has two lone pairs of electrons. This arrangement uses all twelve valence electrons and gives both oxygen atoms a full octet, making the molecule stable.
O₂ has a double bond because each oxygen atom needs two more electrons to reach eight in its outer shell. By sharing two pairs of electrons, both atoms satisfy the octet rule without gaining or losing electrons, creating a stable covalent bond.
O₂ has a total of twelve valence electrons. Each oxygen atom contributes six valence electrons. These electrons are used to form two shared pairs in the double bond and four lone pairs, completing the Lewis structure.
No, O₂ does not violate the octet rule. In the Lewis structure, each oxygen atom ends up with eight electrons around it. The shared electrons in the double bond count toward both atoms, allowing each to reach a stable configuration.
Oxygen is shown with lone pairs because not all valence electrons are used for bonding. Each oxygen atom has two lone pairs that remain on the atom. These electrons affect reactivity and must be shown for the structure to be accurate.
The basic O₂ Lewis structure stays the same when oxygen is alone. However, during chemical reactions, bonds can break or rearrange. While the structure explains O₂ itself, new structures form when oxygen reacts with other substances.
O₂ has a simple double bond between two oxygen atoms. O₃, known as ozone, has three oxygen atoms and uses resonance structures. Ozone’s Lewis structure is more complex and shows alternating single and double bonds.
O₂ is a diatomic molecule, meaning it contains only two atoms. Because of this, it has a linear shape with a bond angle of 180 degrees. There are no bends or angles beyond the straight-line arrangement.
Each oxygen atom has an electron configuration ending in six valence electrons. In the O₂ molecule, these electrons are shared through a double bond. Advanced models show unpaired electrons, explaining oxygen’s paramagnetic behavior, even though the Lewis structure simplifies this view.







